Pandemic Response
What was the immediate impact of freezing USAID funding during a health crisis?
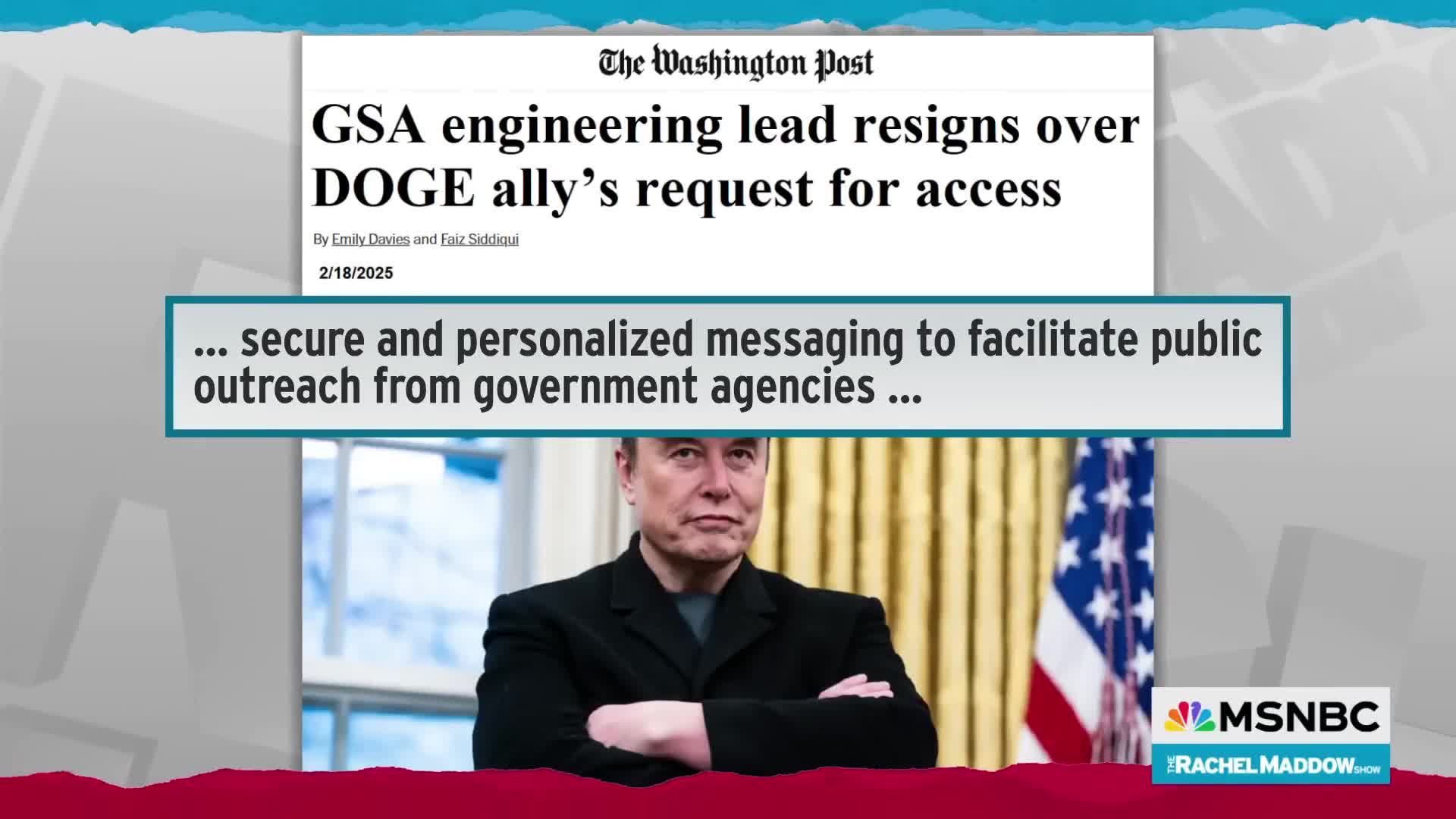
The impact of freezing USAID funding was immediate and far-reaching beyond just Ebola and HIV programs. Dr. Spencer emphasizes that USAID serves as 'our eyes and ears' in countries like Uganda and Rwanda, establishing crucial relationships and surveillance networks. When Elon Musk announced they would 'throw USAID into the wood chipper,' it disrupted these established partnerships that enable disease detection and response. This disruption weakens global health security infrastructure at a critical time when international collaboration is needed to monitor and contain outbreaks, making communities worldwide more vulnerable to emerging health threats.
Watch clip answer (00:18m)What unexpected situation occurred with bird flu experts during the Trump administration?
According to NBC News, the Trump administration accidentally fired experts working on bird flu at the USDA. This appears to have been an unintended action, with Rachel Maddow sarcastically noting they "totally didn't mean to fire" these specialists. The administration is now reportedly attempting to rehire these experts after recognizing the error. This incident follows a similar pattern with the National Nuclear Security Administration, which Maddow had discussed previously. These actions represent concerning lapses in maintaining expertise in critical public health and safety areas, particularly regarding pandemic preparedness during a time of heightened concern about infectious diseases.
Watch clip answer (00:36m)What is happening with the frontline workers responding to the bird flu outbreak?
Frontline safety workers responding to the bird flu outbreak were fired over the weekend, prompting immediate government action. The Department of Agriculture has acknowledged the situation and stated they are working to swiftly rectify these dismissals of employees who are considered essential to the public health response. The government is now actively trying to get these workers back, recognizing their critical role in managing the outbreak. This incident highlights tensions within federal workforce management during a public health situation, particularly concerning essential personnel responsible for disease containment and safety protocols.
Watch clip answer (00:14m)Why does Congresswoman Crockett believe daily briefings are needed to combat misinformation?
During the COVID-19 pandemic, daily briefings became essential because the public couldn't trust Donald Trump's statements about the pandemic, including dangerous suggestions like injecting bleach. Instead, people turned to what Crockett describes as a 'shadow president' who provided factual information. Crockett advocates for implementing a similar approach today because Trump and his allies, including his 'new press secretary' (likely referring to Elon Musk), will continue to 'spew lies upon lies upon lies.' Regular, fact-based briefings would provide Americans with reliable information to counter this persistent stream of misinformation.
Watch clip answer (00:25m)How are workforce cuts in public health agencies impacting our ability to respond to disease outbreaks?
Current major cuts to public health agencies are being implemented in an arbitrary manner rather than based on actual needs, with Dr. Michael Osterholm describing it as using a 'machete' where 'surgical instruments' would be more appropriate. These workforce reductions are particularly concerning because they're forcing out young professionals who represent the future leadership of these organizations. The consequences of these cuts won't be temporary but will negatively impact public health capacity and disease response for an entire generation, undermining both present capabilities and future preparedness.
Watch clip answer (00:50m)What does Jumaane Williams think about Andrew Cuomo potentially running for mayor of New York City?
Jumaane Williams expresses uncertainty about which would be worse - Mayor Adams or Mayor Cuomo - highlighting a concerning leadership situation in NYC. He observes that people often confuse bullying with leadership, noting that some might be drawn to Cuomo's style based on current political dynamics. However, Williams cautions against selective amnesia, urging New Yorkers to remember how harmful Cuomo was in many of his decisions, particularly during and after the COVID pandemic. His assessment reflects deeper concerns about the quality of leadership facing the city and the potential consequences of electing someone with Cuomo's governing history.
Watch clip answer (00:27m)