Medication Safety
What challenges do COVID-19 vaccine trial participants face when experiencing adverse effects, and how have authorities responded to vaccine injury claims?
Brianne Dressen's experience highlights significant challenges faced by vaccine trial participants who suffer adverse effects, including difficulties gaining recognition from medical authorities and fighting for accountability. Her journey from trial participant to advocate reveals systemic issues in how vaccine injuries are acknowledged and addressed by healthcare institutions and government organizations. The discussion exposes broader problems with COVID-19 vaccination protocols and the limited options available for those experiencing similar complications. Despite emerging evidence that vaccines don't prevent Long Covid symptoms, affected individuals often struggle to receive proper medical support and official recognition of their vaccine-related health issues.
Watch clip answer (00:07m)What challenges do vaccine trial participants face when experiencing adverse effects, and how does this impact accountability in the pharmaceutical industry?
Based on Brianne Dressen's experience as an AstraZeneca COVID-19 vaccine trial participant, individuals who suffer severe adverse effects often encounter significant challenges including lack of support from trial organizers and limited transparency in the injury reporting process. Her advocacy work highlights systemic issues where vaccine-injured participants struggle to receive recognition, proper medical care, and compensation for their injuries. This raises critical concerns about pharmaceutical accountability and the need for better support systems for trial participants who experience unexpected health consequences during vaccine development and testing phases.
Watch clip answer (00:04m)How do compounding pharmacies operate under FDA regulations, and why are pharmaceutical companies trying to restrict their ability to compound certain medications?
Compounding pharmacies are authorized by the FDA to create medications when there's an official shortage or "backlog" of approved drugs. They can use the same molecules as patented drugs but modify delivery systems and dosages to serve patients when branded medications are unavailable. However, major pharmaceutical companies like Eli Lilly are using their political influence to pressure the FDA to remove drugs from the shortage list, even when they cannot meet market demand. This creates a conflict where life-saving emergency medications (like crash cart drugs in hospitals) rely heavily on compounding pharmacies due to low profit margins, yet Big Pharma seeks to eliminate this competition to expand their market control.
Watch clip answer (02:05m)How did the opioid crisis develop through big pharma corruption and over-prescription practices?
The opioid crisis emerged through systematic corruption within the pharmaceutical industry, where companies prioritized profit over patient welfare. Healthcare expert Brigham Bueller explains that pharmaceutical companies deliberately incentivized the over-prescription of opioids, creating widespread addiction and devastating community impacts. This crisis represents a clear example of how corporate greed can corrupt healthcare systems, leading to one of the most significant public health disasters in recent history. The discussion emphasizes the urgent need for healthcare reform and corporate accountability to prevent similar tragedies.
Watch clip answer (00:23m)What do President Trump's health department appointments signal about his administration's approach to COVID-19 policies and pandemic response?
Trump's proposed health appointments, particularly Dr. Jay Bhattachary for the National Institutes of Health, strongly indicate his administration plans to reexamine the entire COVID-19 pandemic response. These nominations suggest a critical review of previous policies including lockdowns and vaccine mandates, with Bhattachary being noted as a vocal COVID critic. The appointments reflect broader public concerns, with 25% of Americans believing they know someone killed by COVID vaccines. This signals Trump's administration will likely investigate pandemic origins and policies through congressional oversight, emphasizing transparency in government health decisions as America continues navigating pandemic recovery complexities.
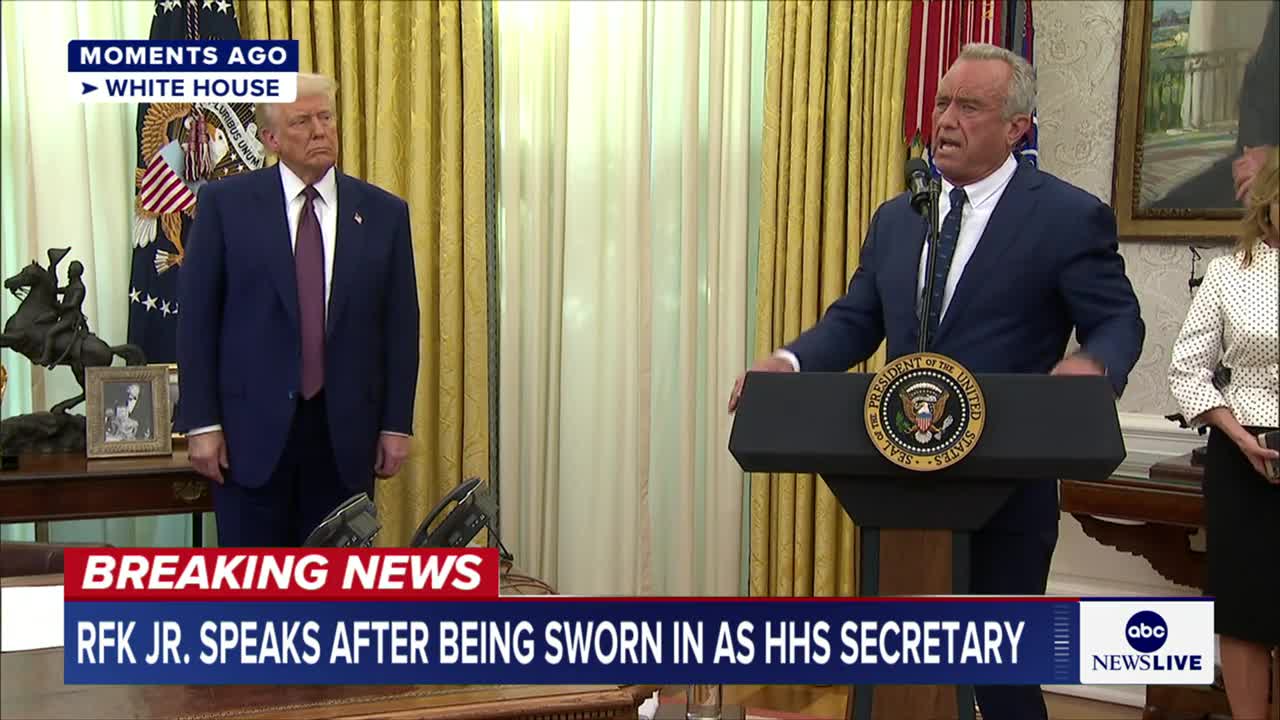
Watch clip answer (00:45m)What are Robert F. Kennedy Jr.'s immediate priorities and potential policies as the newly sworn-in Secretary of Health and Human Services?
Robert F. Kennedy Jr.'s primary focus as HHS Secretary centers on addressing America's childhood chronic disease epidemic through his "Make America Healthy Again" initiative. His immediate plans include establishing a MAHA commission to investigate health issues and potentially cutting federal funding to schools that mandate vaccinations, reflecting his long-standing skepticism about vaccine safety and efficacy. Kennedy also intends to scrutinize food safety regulations, particularly examining additives in American food products that he believes may contribute to health problems. His approach involves demanding more comprehensive studies on substances currently deemed "generally recognized as safe" by food manufacturers, positioning himself as a reformer challenging established health policies and industry practices.
Watch clip answer (02:34m)